Dedicated to
assisting
your patients
With DARAPRIM Direct, your patients will have a dedicated DARAPRIM Direct Care Coordinator to assist them through every step.
To learn how to prescribe DARAPRIM, click below.
Accessing DARAPRIM
Healthcare Provider

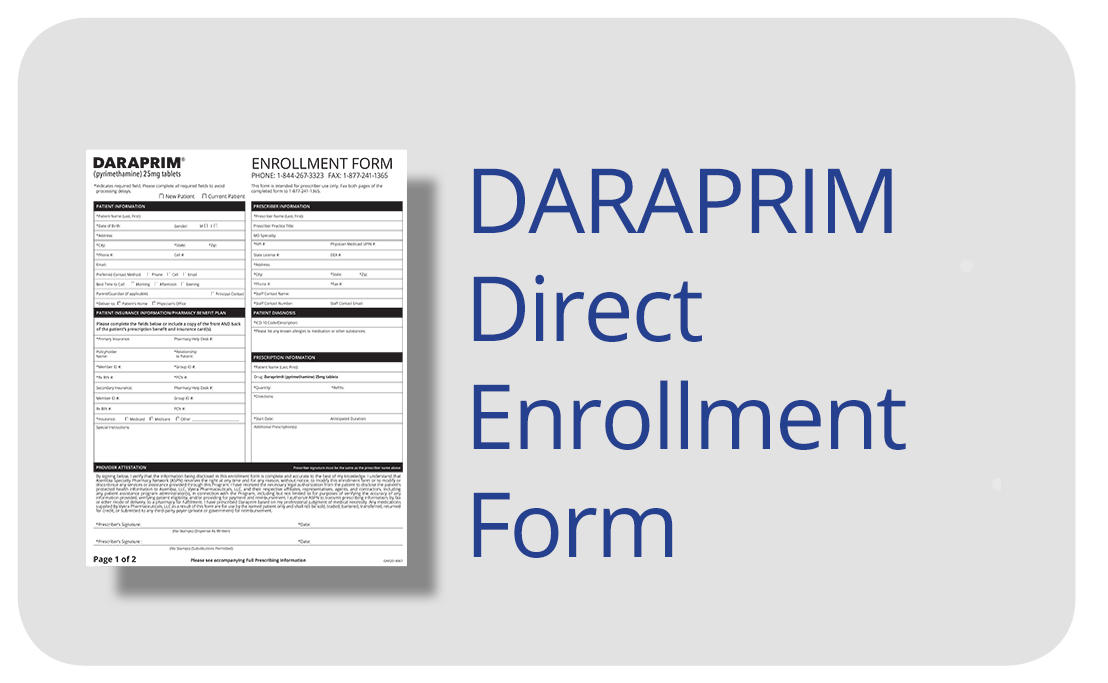
Step 1: Download and print the DARAPRIM Direct Enrollment Form.

Step 2: Fill out the form with all indicated information.

Step 3: Fax the form(s) to

Step 4: Inform your patient that DARAPRIM Direct will be calling them to schedule their medication delivery.
If you have questions, call

Step 1: Download and print the DARAPRIM Direct Enrollment Form.

Step 2: Fill out the form with all indicated information and make sure to have your patient sign the patient authorization section

Step 3: Fax the form(s) to

Step 4: Inform your patient that DARAPRIM Direct 5will be calling them to schedule their medication delivery
If you have questions, call
Health System

Contact DARAPRIM Direct by calling

Contact DARAPRIM Direct by calling
OR

Order by emailing:
asd.customerservice@asdhealthcare.com